| |
|
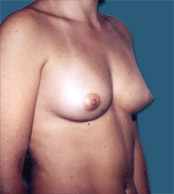
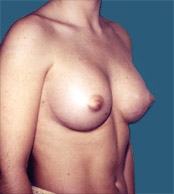
Breast Implants are used during Plastic Surgery to give breasts a different shape and or volume than that before. In the case of Reconstructive Surgery breast implants are frequently used after a mastectomy (removal of one or both breasts).
 |
 |
| Before |
After |
There are two ways to put the implants in place: either behind the « grand pectoral » muscle, or behind the gland and therefore in front of the muscle.
The implant behind the muscle is more painful after the operation. This is due to the permanent massage of the muscle that notably diminishes the possibility of a periprothetic shell, i.e. in 3 to 6% of the cases there is a fibrosis reaction which hardens the tissues when it comes into contact with the implants’ envelope. This hardening of the tissues can be invisible and fine to the touch, but it can also be very intense and at the last resort it can diminish the breasts’ movement and even freeze it. This would then require another surgery. Nevertheless, in specific cases it is possible to place the implants behind the mammary gland.
Up until May 1995 no legislation on Mammary implants existed.
In fact, the practitioner had the choice between various implants: there were those filled with silicone gel, those with hydrogel (water-soluble gel) or those filled with saline solution. All without exception have a silicone envelope. The manufacturers of these implants have always maintained a very high quality and manufacturing standard (i.e. the purity of the materials used, sterilisation).
In 1979 in the USA, the all powerful Food and Drug Administration, whose seal of authorisation is required in order to be allowed to place a pharmaceutical product on the market, had asked for further studies.
Still in the USA, in 1990 during the TV Talk Show “Face to Face” the breast implants were presented as part of the “horror stories of Plastic Surgery”. This was the start of a serious campaign against silicone gel.
In France, it was only at the end of 1994 that the French Ministry of Health installed an approval system via a number code for each type of implant.
As of May 1995 still in the USA, after an information campaign on some of the pathological cases that were due to the usage of silicone, the only implants that were authorised were those filled with saline solution.
The usage of all other implants was suspended for a year, but this was then extended for further information.
Nevertheless, patients who have benefited from the silicone filled implants prior to the suspension should be reassured. In fact since June 2001 France has once more authorised their usage.
It is the same for all those who have breast implants: they must have yearly check ups to control if there are any possible leaks, as well as to check the possible formation of an outer periprothetic shell. However, it must be said that all silicone injections remain forbidden, as for example in the case of filling of lips.
 Top of the page Top of the page
|